
- Pipe
- Bridges & Structures
- Walls
- Stormwater Management
- Erosion Control
- Start a Project
- Knowledge Center
- Technical Documents
In many parts of the United States, stormwater quality regulations are expanding to include treatment criteria for total phosphorus. Phosphorus is the second-most-regulated pollutant in stormwater runoff after total suspended solids. However, engineers and regulators are still learning how to remove phosphorus from stormwater to promote healthy waterways and meet agency requirements. This article will provide an overview of the current state of phosphorus treatment research and how this research can help guide decisions when specifying stormwater Best Management Practices (BMPs).
Phosphorus is a naturally occurring element and a nutrient necessary for the health of all forms of biological life. In the aquatic environment, elemental phosphorus is rarely found by itself; it is typically present as a compound. It can be in inorganic and organic forms, and it can be found attached to particulate matter or in dissolved forms. Adding to the complexity, phosphorus can shift forms between being dissolved and attaching to particulate matter within the water stream as it reacts to other compounds and variables in stormwater runoff, such as dissolved oxygen.
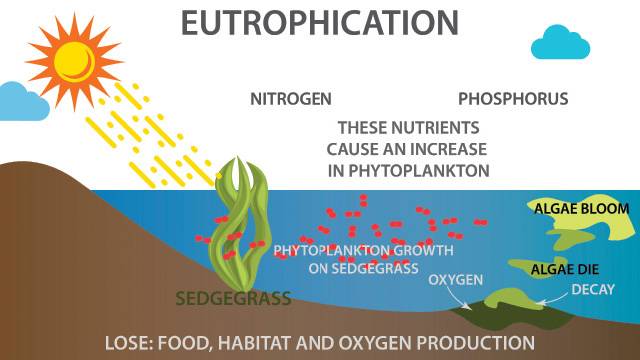
Phosphorus also is often the limiting nutrient for organisms in freshwater and can be a primary cause of eutrophication and harmful algal blooms (HAB) (Figure 1). The U.S. Environmental Protection Agency identifies HABs as a major environmental problem in all 50 states. Eutrophication occurs when the aquatic environment becomes enriched with nutrients, increasing plant and algae growth. The excess algae and plant matter eventually decompose, reducing oxygen to levels that can kill fish and seagrass as well as reduce essential habitats, often resulting in mass die-off events. This growth also limits light penetration, which hinders the development of ecologically beneficial plants.
The chemical form of phosphorus (also known as speciation) significantly impacts how the presence of phosphorus affects the water body. For example, dissolved phosphorus is more readily available to algae than particulate-based phosphorus, making dissolved phosphorus concentrations more directly relevant to eutrophication and HABs.
Sources of phosphorus in urban runoff include compost, manure, plant and leaf litter, soil particles, pet waste, road salt, fertilizer, and atmospheric deposition of particles. Lawns and roadways account for the most-significant loading.
In stormwater runoff, phosphorus typically is measured by Total Phosphorus (TP), Total Dissolved Phosphorus (TDP) and Orthophosphate (OP). TP includes both particulate-bound and dissolved phosphorus. Particulate-bound phosphorus is typically attached to small silt and clay particles due to the high positive charge and large surface area of small particles. TDP is denoted by particle size, and, in stormwater chemistry, the dissolved phase is separated by passing a sample through a 0.45-micron filter. OP is the simplest form of inorganic phosphorus and is immediately available for uptake by algae and aquatic plants. OP typically is measured using colorimetry.
Research is ongoing, but current studies show the ratio of TP to TDP ranges from 20 to 90 percent. The wide range is primarily due to source control, land use, seasonality and sampling location within the watershed. These variables also can influence the OP fraction of TDP.
Given that we know phosphorus exists in different species, success in treating phosphorus is dependent on which forms are regulated, present and being analyzed.
The speciation of phosphorus previously discussed makes it challenging to remove from urban runoff.
Dissolved phosphorus is much more difficult to remove than particulate-bound phosphorus because it relies on different treatment mechanisms. Gravity separation and filtration can physically remove particulate-bound phosphorus; however, dissolved forms require chemical removal processes such as adsorption or precipitation.
Further, while a portion of the phosphorus load may be bound to particulates, it may not stay that way, as it can dissociate into the dissolved phase. For example, phosphorus attached to organic compounds in leaves can transform into dissolved phosphorus as the leaves decompose. This phenomenon can be observed in the spring and fall when flowers and other plants die and decompose. It can explain why seasonality affects the ratio of TP to OP, and why algae blooms within water bodies are more frequent during certain seasons.
Meeting most TP removal goals often means treating both the particulate-bound and dissolved phosphorus phases as well as retaining any captured particulate phosphorus that may dissociate into the dissolved phase. Standard stormwater treatment practices adequately remove particulate-bound phosphorus from urban runoff. However, removing and retaining dissolved phosphorus is more complex and requires specialized treatment.
Many state regulatory agencies have established phosphorus removal targets (Figure 2). Most of these agencies structure their removal goals as a percentage removal of TP, which underscores the importance of knowing the dissolved fraction of TP.
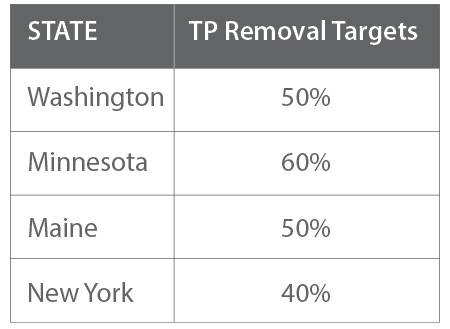
Geography has a significant impact on a state’s phosphorus-removal targets. For example, Minnesota has more freshwater bodies than Arizona and thus has more-stringent removal goals. Land use also is critical. Agricultural runoff significantly contributes to high phosphorus levels, but it’s regulated differently from urban areas. States with overlapping agricultural and rural land uses may have to coordinate among various agencies to meet phosphorus-removal goals.
Phosphorus also is a pollutant of concern in section 303(d) of the Clean Water Act. It leads the nutrient category in the Total Maximum Daily Load (TMDL) category, with more than 4,000 water bodies listed as impaired by TP.
Phosphorus also is mobile, with large multi-state drainage basins showing higher phosphorus concentrations downstream. This can lead to permanent areas of low dissolved oxygen—also known as dead zones—and has led to the development of large, regional approaches such as the Chesapeake Bay TMDL, which covers six states. Lastly, some states, such as Virginia, have instituted nutrient credit trading to control stormwater phosphorus levels.
Phosphorus typically is removed from stormwater using filtration or bioretention/biofiltration.
Filtration
Filtration processes to remove phosphorus can be split into two groups based on the type of media used: 1) physical/inert media filtration and 2) active/sorptive media filtration.
In physical/inert media filtration, particulate-bound phosphorus is screened out of suspension using a physical barrier. Examples include horizontal bed filters, sand filters, bulk cartridge filters and membrane filters (Figure 3). However, there is no mechanism to remove dissolved phosphorus.

Active/sorptive media filtration offers the same physical processes as inert filtration, with the addition of a passive chemical process to remove dissolved phosphorus via adsorption, ion exchange or surface precipitation. This treatment utilizes materials such as activated carbon, biochar, activated alumina or iron (Figure 4).

Bioretention/Biofiltration
Bioretention has been the principal form of Low-Impact Development (LID) used to slow, treat, retain and infiltrate stormwater runoff, mimicking a site’s natural, pre-development hydrology.
Bioretention systems use vegetation and engineered soils to capture and filter stormwater runoff, contributing to the uptake of pollutants and runoff volume reduction. As runoff percolates through the bioretention media, sediments and pollutants undergo physical (e.g., filtration), biological (e.g., plant uptake, microbial denitrification) and physicochemical (e.g., removal of dissolved phosphorus through sorption) processes. Other systems that use this process include rain gardens, vegetated filters, bioswales, biofilters and tree-box filters.
Nutrient Leaching
While bioretention/biofiltration effectively removes phosphorus, there is a potential for nutrient leaching. Nutrient leaching occurs when bioretention media, which often contains compost, breaks down and releases phosphorus to downstream receiving waters.
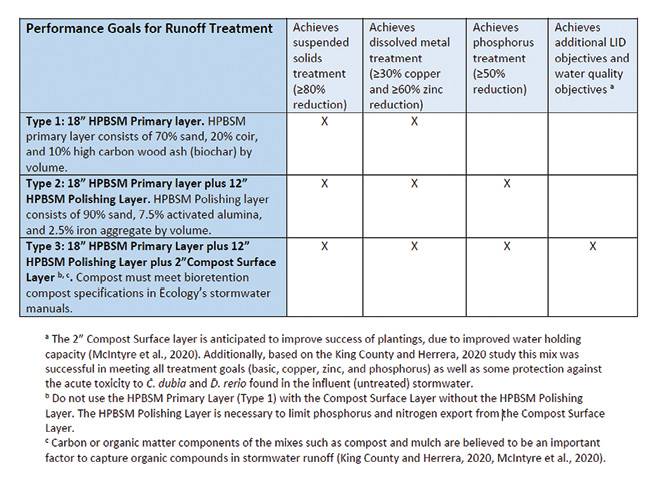
Studies in Washington have documented the release of phosphorus and copper from installations using the state’s standard 60/40 sand/compost blend specified for bioretention systems. The problem has resulted in Washington’s Department of Ecology issuing a guidance document with a new set of media specifications for bioretention media depending on their proximity to phosphorus-sensitive receiving waters (Figure 5). The revised specifications call for removing/limiting the amount of compost allowed and replacing it with coir (coconut fiber), biochar (wood ash) and other amendments. The specification also calls for polishing layers containing an activated media, including alumina and iron aggregate. While effective, these media blends may be difficult to source and install properly on a consistent basis. Verification and toxicity testing of the media components may be needed to ensure these systems function as designed, further adding to these systems’ cost and complexity.
Performance Data on Phosphorus Removal
The International Stormwater BMP Database (BMP Database)4 is a repository of BMP field studies and related web tools, performance summaries, and monitoring guidance. Managed by The Water Research Foundation, the project’s purpose is to provide field-monitoring data to improve the design, selection and performance of urban stormwater BMPs.
Most of the manufactured treatment system data included in the BMP Database comes from the State of Washington’s Technology Assessment Protocol – Ecology (TAPE) program, the most widely recognized program for evaluating treatment devices through field monitoring of full-scale systems. The protocol requires an evaluation of numerous storm events and sets a rigorous standard for removing stormwater pollutants. Systems that complete the protocol are approved by the state and may be specified to meet pollution-reduction goals. Dozens of states and local jurisdictions also recognize the TAPE verification program and will reciprocate approvals for devices that have met the TAPE standards.
The BMP Database’s latest summary statistics report was published in 2020 and includes new BMP categories, including high-rate biofiltration and high-rate media filtration.
Sediment/Total Suspended Solids Removal (TSS)
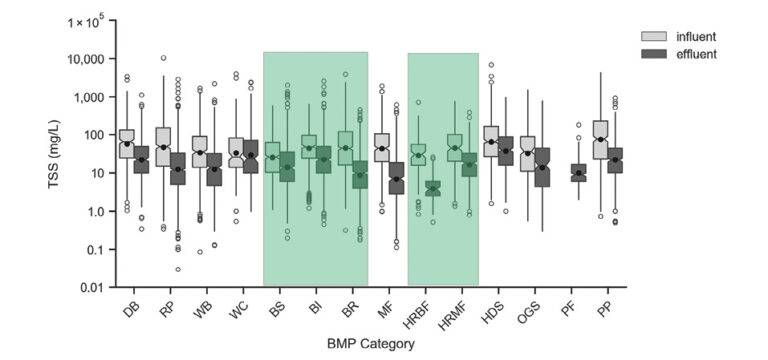
The box plot shown in Figure 6 shows the difference in TSS influent and effluent concentrations of stormwater treated by different BMPs. Highlighted in green on the left are the results for conventional green infrastructure such as grass swales (BS), grass strips (BI) and bioretention (BR). Highlighted on the right are high-rate biofiltration (HRBF) and high-rate media filtration (HRMF) results. The results show that these practices do a good job removing total suspended solids, as demonstrated by the lower effluent concentrations.
Total Phosphorus Removal (TP)
The data for TP removal tells a very different story. Traditional green infrastructure such as grass swales (BS), grass strips (BI) and bioretention (BR) shown in Figure 7 all demonstrate an export of phosphorus, as shown by the higher effluent results. This means these systems are likely exporting phosphorus from the media, or particulate phosphorus is desorbing from the TSS and passing downstream. Either way, this is troubling as stormwater BMPs are implemented to remove pollutants from stormwater, not contribute them to receiving waters.
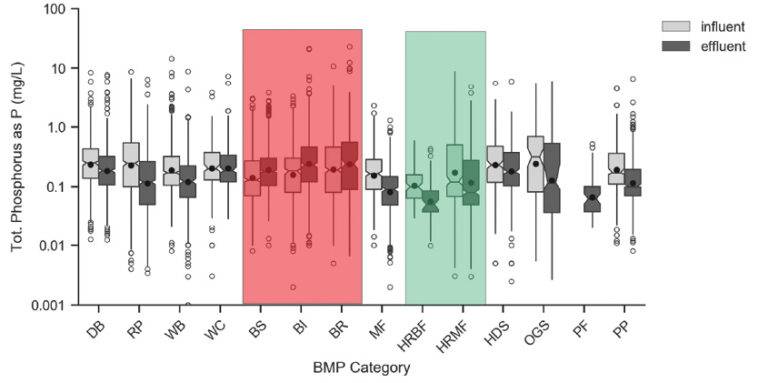
The results for high-rate biofiltration (HRBF) and high-rate media filtration (HRMF) are quite different. Both systems consistently remove TP, as shown by the significantly lower effluent levels.
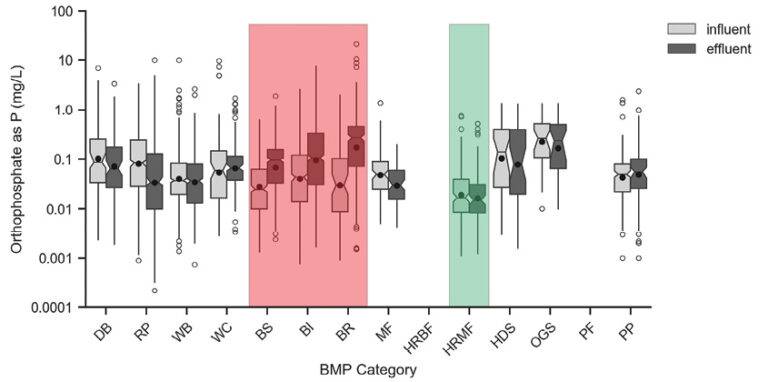
Orthophosphate Removal (OP)
Figure 8 indicates that traditional green infrastructure show a significant export of OP, as indicated by the higher effluent levels. Data from high-rate media filtration show that while there was no significant removal of OP, there was also no export of captured phosphorus. At the time of publication, there was not enough data to meet the summary statistics requirements for high-rate biofiltration.
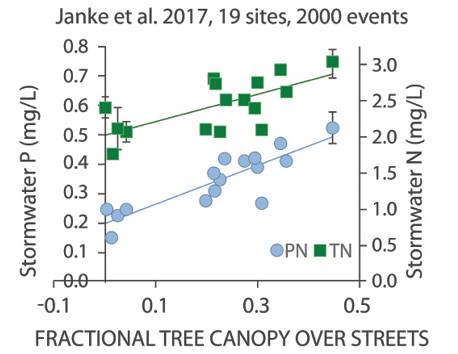
Timing
Every stormwater BMP will need maintenance to ensure the system functions as designed, and the timing of maintenance can affect the annual cumulative load reduction. For example, research by Janke et al. and the University of Minnesota (Figure 9) suggests tree canopy cover and corresponding leaf drop during the fall can play a significant role in influent total phosphorus concentrations, with phosphorus in stormwater increasing as the percentage of urban tree canopy increases. This suggests timing BMP maintenance to the late fall to capture leaf drop before its decay may be a best practice. Similarly, ice covers and deicers can reduce available oxygen in cold-weather climates as well as increase the amount of dissolved phosphorus in snowmelt and spring runoff. Therefore, maintenance timing also may be best after the spring snowmelt to help achieve desired phosphorus-removal results.
Type of Maintenance
The type of maintenance conducted also plays a role in phosphorus reduction. For example, when maintenance is done on high-rate active-media filtration systems, the entire filter unit typically is replaced, permanently removing the captured pollutants from the waste stream. Membrane filtration also can permanently remove phosphorus from the waste stream, but this depends on how well and how often filter cartridges are rinsed and replaced.
Biological systems such as rain gardens, traditional bioretention and high-rate bioretention systems rely on microbial transformation and biological assimilation to remove dissolved phosphorus from the waste stream. Many of these systems also utilize mulch as a pretreatment mechanism. When the mulch is removed and replaced during a maintenance event, a large amount of sediment and particulate-bound phosphorus can be permanently removed from the waste stream.
BMPs such as sand filters, grass strips and swales are more challenging to maintain as it relates to phosphorus removal. For example, a sand filter will only remove particulate-bound phosphorus if the top level of sand is removed and replaced; and there is no biological removal process to manage the dissolved phosphorus. As a result, some have turned to amending sand filters to include additives such as iron filings to improve phosphorus removal. To achieve long-term performance, the spent iron additive must be removed and replaced, which typically requires removing the entire sand filter media and may not be feasible.
The effects of excess phosphorus in stormwater can cause increased growth of algae and other aquatic plants, resulting in algae blooms and decreased levels of dissolved oxygen that can be harmful to human, animal and ecological health. However, phosphorus is challenging to remove from stormwater, as it exists in both particulate-bound and dissolved forms.
Studies have shown that traditional bioretention practices can potentially export phosphorus downstream of receiving waters and contribute to the problems they are meant to solve. Data from high-rate media filters and high-rate biofiltration systems demonstrate the removal of total phosphorus without this leaching effect. To meet or exceed phosphorus-removal goals and remove phosphorus from the waste stream, specifying engineered media blends such as high-rate media filtration or high-rate biofiltration may provide a better solution than traditional practices such as conventional bioretention or sand filters.
• https://www.epa.gov/nutrientpollution/harmful-algal-blooms
• https://apps.ecology.wa.gov/publications/documents/1310017.pdf
• https://apps.ecology.wa.gov/publications/documents/2110023.pdf
Click on the button below to start the quiz for this course. Your score will be tabulated while you wait, and you will receive your certificate upon completion if you correctly answer eight or more questions.
Registration on v1-education.com is required to access the quiz. Use the "Sign Up" link in the top right of v1-education.com to register. If you are already registered simply enter your credentials to access the quiz.
The Professional Development Series is a unique opportunity to earn continuing education credit by reading specially focused, sponsored articles in Informed Infrastructure.
If you read the following article, display your understanding of the stated learning objectives, and follow the simple instructions, you can fulfill a portion of your continuing education requirements at no cost to you. This article also is available online at v1-education.com.
At the conclusion of this article, the reader should be able to understand:
Click on the button below to start the quiz for this course. Your score will be tabulated while you wait, and you will receive your certificate upon completion if you correctly answer eight or more questions.
Registration on v1-education.com is required to access the quiz. Use the "Sign Up" link in the top right of v1-education.com to register. If you are already registered simply enter your credentials to access the quiz.